Abstract
Introduction We have reported that patients with myeloid malignancies were more responsive to the second dose of mRNA-based COVID-19 vaccines than patients with lymphoid malignancies (Mori, et al. Br J Haematol. 2022;197:691.). However, there are very limited data of response for a third vaccine dose in these patients. In this study, we investigated the antibody titers of COVID-19 in patients with myeloid malignancies who received a third mRNA-based COVID-19 vaccine dose (booster).
Patients and Methods Previously treated, currently treated, and newly diagnosed myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) patients were included in this study. All patients were vaccinated with at least two doses of mRNA-based COVID-19 vaccine, either BNT162b2 or mRNA-1273, and visited the Blood Disorders Center at Aiiku Hospital during the period from August 17, 2021 to July 15, 2022. We recruited healthcare workers aged 50 years and older who had received at least two doses of BNT162b2 vaccine as healthy controls (HCs). Anti-SARS-CoV-2S immunoassays were performed at 3 months ± 2 weeks, 6 months ± 4 weeks, and 9 months ± 4 weeks after the second vaccine dose. All third doses were administered between the 6-month and the 9-month blood samplings. Individuals who did not received a third dose prior to the 9-month blood sampling were excluded from the booster effect analysis. Individuals with a known history of COVID-19 were excluded from both cohorts of patients and HCs. This study was part of a prospective observation study (UMIN000045267) and was conducted in compliance with ethical principles based on the Helsinki Declaration and the study was approved by the institutional review board of Aiiku Hospital.
Results A total 58 patients with myeloid malignancies with a median age of 70.5 (range: 18-88) years including 20 patients with MDS and 38 patients with AML were enrolled. HCs includes 29 individuals with a median age of 55.0 (range: 50-72) years. Fourteen patients were receiving active treatment, one patient had terminated active treatment, 4 patients were treatment-naive, and one patient had not yet been diagnosed at the time of initial vaccination in MDS patients. Thirteen patients were receiving active treatment, 22 patients were under treatment-free observation in complete remission after treatment, and 3 patients had not yet been diagnosed at the time of initial vaccination in AML patients.
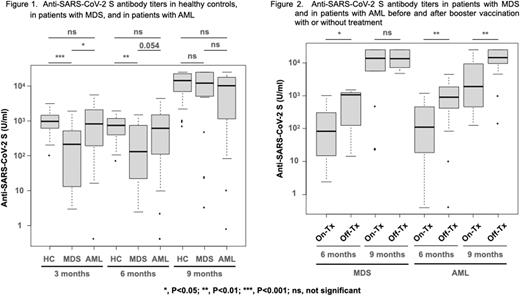
Seroconversion rates after the second vaccination for HCs, MDS patients, and AML patients were 100%, 100%, and 94.7%. Patients with MDS showed a significantly lower antibody titer after second vaccination than that in HCs or AML patients, and there was no significant difference between the antibody titers after second vaccination in HCs and AML patients (Figure 1). Third vaccination rates were 89.7%, 95.0%, and 89.5% in HCs, MDS patients, and AML patients, and antibody titers increased prominently after booster vaccine, respectively (Figure 1). Furthermore, the antibody titer in MDS patients increased to comparable levels to that in HCs or AML patients after the third vaccine booster (Figure 1).
In MDS patients, the median antibody titer before the booster vaccine was significantly lower in patients on active treatment than in patients under treatment-free or treatment-naive (88.0 U/ml vs 1076.5 U/ml, p<0.05) (Figure 2). However, the median antibody titer after the booster vaccine in MDS patients who were receiving active treatment at the time of the third vaccination increased to the comparable levels to that in MDS patients who were under treatment-free or treatment-naive (Figure 2). On the other hand, AML patients on active treatment at the time of third vaccination showed a significantly lower antibody titer after booster vaccination than that in AML patients under treatment-free observation (p<0.01) (Figure 2). In AML patients actively treated with hypomethylating agent (HMA) ± venetoclax at the time of the third dose, the median antibody titer after booster vaccine was significantly lower than in the other patients (HMA; p<0.05, HMA + venetoclax; p<0.05).
Conclusion The third dose of mRNA-based COVID-19 vaccine showed the remarkable booster effect in patients with myeloid malignancies. Especially, the booster vaccine brought a significant improvement in response to MDS patients who had shown inferior response after two doses of vaccination, even to patients who were receiving active treatment.
Disclosures
Teshima:Fuji Pharma: Research Funding; TEIJIN PHARMA: Research Funding; Astellas: Research Funding; Chugai: Research Funding; Bristol-Myers Squibb: Honoraria; Merck Sharp & Dohme: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Research Funding; NIPPON SHINYAKU: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Manuscript preparation, Research Funding; Janssen: Other: Manuscript preparation; Pfizer: Honoraria; Kyowa Kirin: Honoraria, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Luca Science Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal